Abstract
Background Paroxysmal nocturnal hemoglobinuria (PNH) is a rare, acquired disease characterized by complement-mediated hemolysis and thrombosis. It results from loss of the glycosylphosphatidylinositol-linked complement-inhibitor proteins CD55 and CD59 that inhibit early and late complement proteins, respectively, on red blood cell (RBC) membranes. In untreated patients, formation of the C5b-9 membrane attack complex results in chronic intravascular hemolysis (IVH) and acute hemolytic attacks. Treatment with the C5 inhibitors eculizumab (ECU) and ravulizumab inhibits IVH and unmasks extravascular hemolysis (EVH), which is due to C3b-mediated opsonization of RBCs and their clearance by the reticuloendothelial system. This can result in unconjugated (indirect) hyperbilirubinemia, leading to jaundice and scleral icterus when plasma bilirubin levels reach 2.0-3.0 mg/dL. As in other conditions with EVH (eg, sickle cell disease), bilirubin gallstones have been reported in patients with PNH treated with C5 inhibitors. Pegcetacoplan (PEG), a C3 inhibitor that controls IVH and EVH, is approved in the US for the treatment of adults with PNH. We hypothesized that PEG could control hyperbilirubinemia in PNH patients, reducing jaundice and the risk of gallstone formation.
Methods This post hoc analysis evaluated 16- and 26-week data from 2 Phase 3 randomized controlled trials. PEGASUS (NCT03500549) evaluated patients with hemoglobin <10.5 g/dL despite stable ECU treatment for 48 weeks; patients received PEG or ECU for 16 weeks. PRINCE (NCT04085601) evaluated PEG therapy for 26 weeks in patients who were untreated with complement inhibitors.
Mean and median total and indirect bilirubin values were calculated at baseline and at the end of the treatment period for patients treated with PEG and ECU in PEGASUS and with PEG in PRINCE.
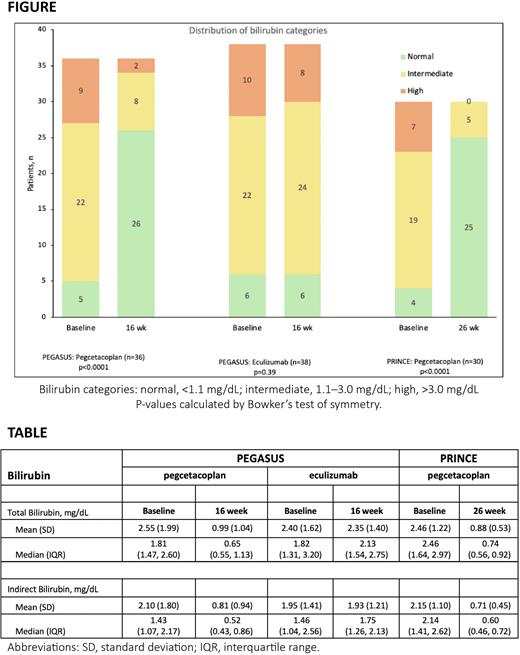
Total bilirubin plasma levels were categorized as normal (<1.1 mg/dL), intermediate (1.1-3.0 mg/dL), and high (>3.0 mg/dL). The main endpoint was the distribution of bilirubin categories at baseline compared with the end of the treatment period and probability of normalization as analyzed by Bowker's test of symmetry.
Patients were excluded from the analysis if they did not have both a baseline and end of treatment bilirubin value for analysis.
Results In PEGASUS, 74 of 80 patients (PEG, n=36; ECU, n=38) had evaluable bilirubin data; 30 of 35 PEG-treated patients were evaluable from PRINCE.
At baseline, 85% (n=63) of patients in PEGASUS had abnormal bilirubin values and 26% (n=20) had high bilirubin values. In PRINCE at baseline, 87% had abnormal bilirubin values, and 23% had high bilirubin values. The hyperbilirubinemia was primarily of the indirect form (TABLE). At 16 weeks, 72% of patients treated with PEG in PEGASUS had normal bilirubin values compared with 16% of patients treated with ECU. In PRINCE, 83% of patients treated with PEG had normal bilirubin at 26 weeks; none had high bilirubin values.
The shifts toward normalization in bilirubin categorization (FIGURE) were statistically significant (p<0.0001) for patients on PEG therapy in both PEGASUS and PRINCE. Continued treatment with ECU was not associated with a significant change in bilirubin (p=0.39).
In PEGASUS, the normalization rate was 71% (n=22/31; exact 95% CI: 52%, 86%) for PEG and 9% (n=3/32; exact CI: 2%, 25%) for ECU. In PRINCE, the normalization rate was 81% (n=21/26 patients; exact CI: 61%, 93%) after PEG treatment.
Conclusions This post hoc analysis found a high prevalence of abnormal bilirubin values at baseline in patients with PNH who were treated with a C5 inhibitor or were untreated. Treatment with PEG significantly improved bilirubin levels, with the majority of patients shifting to normal values. Conversely, bilirubin levels did not improve with continued ECU treatment. These findings suggest that long-term treatment with PEG reduces the signs and symptoms of hyperbilirubinemia, improving jaundice, and decreasing the risk of bilirubin stones and associated complications (eg, biliary sepsis and pancreatitis) in patients with PNH.
Disclosures
Araten:Alexion Pharmaceuticals: Consultancy, Honoraria; Apellis Pharmaceuticals: Honoraria. Yeh:Apellis Pharmaceuticals: Current Employment, Current equity holder in publicly-traded company. Al-Adhami:Apellis Pharmaceuticals: Current Employment. Horneff:Swedish Orphan Biovitrum AB: Current Employment. Grossi:Apellis Pharmaceuticals: Current Employment, Current equity holder in publicly-traded company.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal